Pictures of the Day
4-18-2024
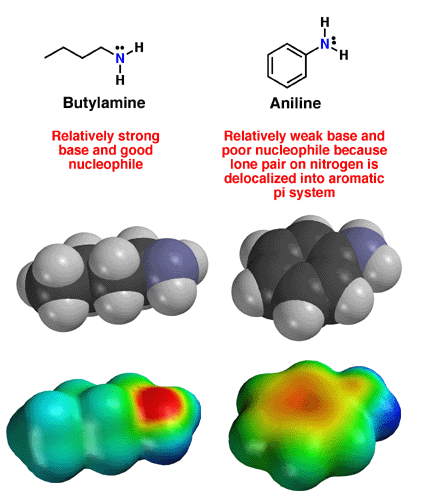
Amine and Aniline Structure
Above are the structures of the butylamine and aniline. Note how the amine nitrogen atom in butylamine has a localized lone pair of electrons. This lone pair is available to interact with Lewis acids such as protons, explaining the red color on the electrostatic potential surface, as well as the fact that amines are good bases and strong nucleophiles. In aniline, the nitrogen atom is planar and thus sp2 hybridized. This occurs because the lone pair of electrons on the nitrogen is now in a 2p orbital, and thus able to delocalize into the aromatic ring. Pi electrons prefere to delocalize whenever possible, so this is a favorable interaction. Note how the electron density of the lone pair, partially delocalized into the pi system of the armatic ring, is less available to interact with protons. As a result, aniline is a much weaker base and nucleophile compared to alkyl amines.
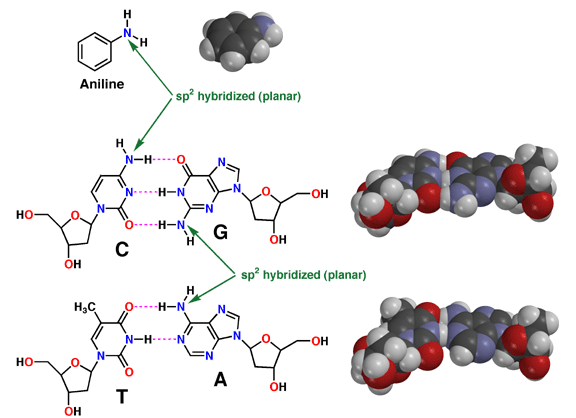
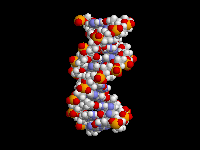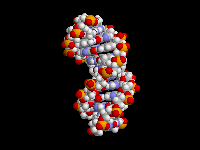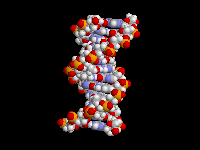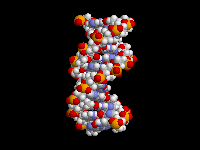
The fact that aromatic amines like aniline are planar and sp2 hybridized is extremely important in biology. There are many biological molecules with aromatic amines, such as the nucleic acid bases. Because these amine groups are planar, the bases can hydrogen bond in the familiar Watson-Crick base pairing arrangement as shown above. In addition, since the entire base is planar, base-pairs can stack like a spiral staircase in the DNA double helix. Thus, the sp2 hybridization of aromatic amines is responsible in large part for one of the most important three-dimensional structures in all of biology.